Usenide® (budesonide) is second generation of corticosteroid and has novel formulation that uses a multimatrix (MMX) technology. this technology is known as colonic delivery technology which permit the budesonide release at control manner throughout the colon and this technology results in the delayed release of drug and a long time in the colon. The tablets are enteric coated to prevent dissolution in the stomach, and do not dissolve until they reach pH ≥7.
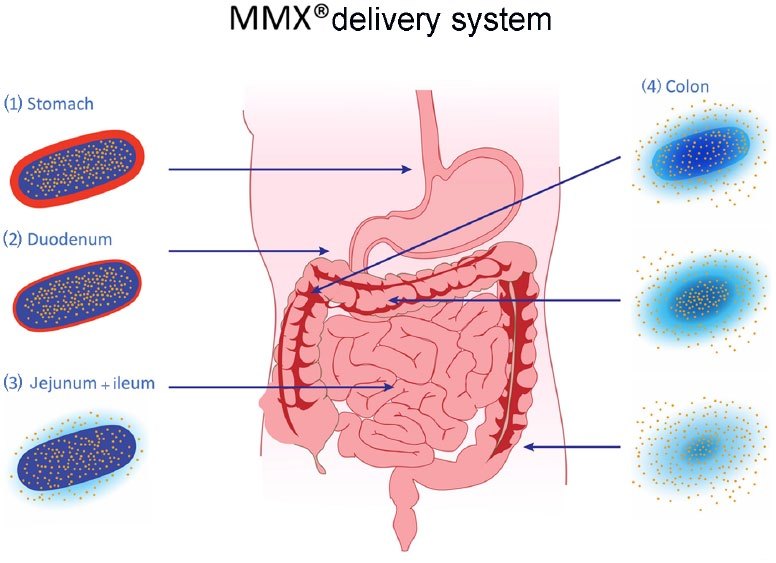
MMX ® technology release throughout the gastrointestinal tract. The gastro-resistant coating (1–2) avoids the release of the drug until the tablet is exposed to a pH ⩾ 7, normally reached in the terminal ileum (3). After reaching this site, the activity of the tablet core results in a homogeneous and prolonged exposure of the whole colonic mucosa to the embedded drug (4)2.
Generic name: budesonide
Brand name: Usenide®
Dosage form: Tablet
Strength: 9 mg
Indication: induction of remission for patients with mild to moderate ulcerative colitis
Dosing per day:
9 mg/day, with or without food in the morning for 8 weeks. It should be swallowed whole and not chewed, crushed, or broken.
Catalog:
Tap the button to view the product catalog.


We will answer any questions you may have.
We are ready to answer right now! Sign up for consultation.
